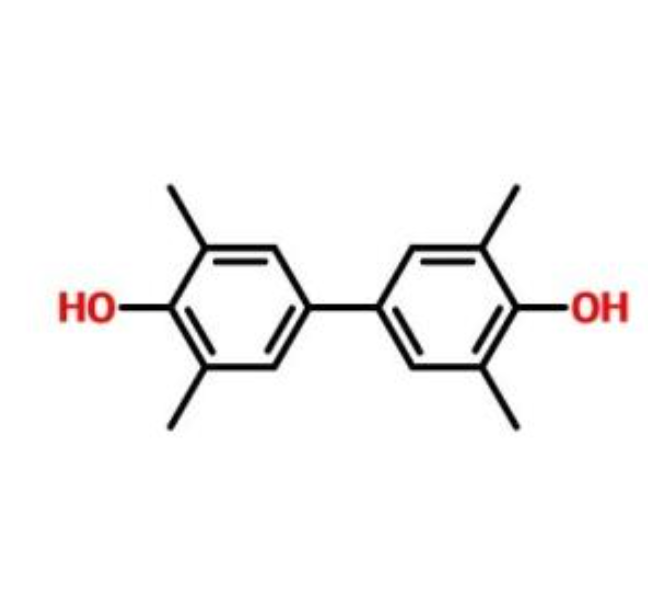
Liquid crystal polymer (LCP) synthesized by 3,3′,5,5′-Tetramethyl-4,4′-biphenyldiol is a common high-performance special engineering plastic, due to its high strength, high modulus, and excellent Dimensional stability, insulation, flame retardancy, low linear expansion rate, radiation resistance, chemical resistance and other comprehensive properties, and are widely used in electronic parts, home appliance parts, medical equipment, precision mechanical parts, automotive parts and components. Chemical equipment parts and other fields also have certain applications in daily chemical products. This article mainly introduces several methods of oxidative coupling to prepare liquid crystal intermediates-3,3′,5,5′-Tetramethyl-4,4′-biphenyldiol (TMBP / TMDHB ).
1. TMBP synthesis method
There are two main routes for synthesizing TMBP in this article.
The base phenol is oxidized to diphenoquinone (DPQ), and then reduced with a reducing agent to obtain the product TMBP. The second is to obtain the product directly by one-step method, but the content of the product is
Impurities such as diphenoquinone and polyphenylene ether are prone to occur and require complicated post-treatment processes, so the first method is more used.
1.1 Synthesize TMBP with oxygen as oxidant
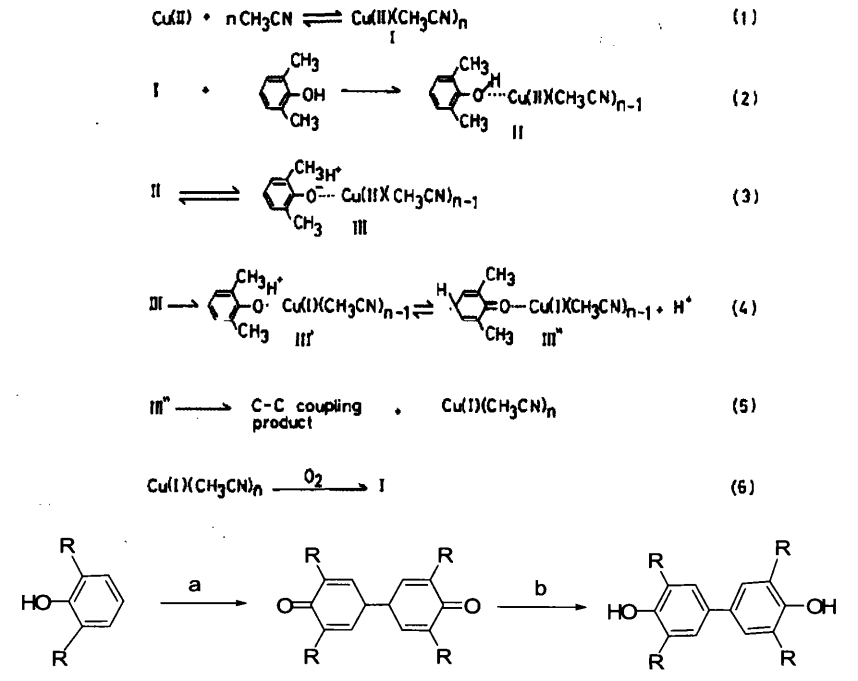
In 1977, ShigeruTsuruya et al. injected oxygen into the copper nitrate-acetonitrile catalytic system to obtain an appropriate amount of quinone and studied the effects of pyridine, piperidine and diethylamine on the oxidative coupling reaction of 2,6-dimethylphenol (DMP). It was found that the addition of pyridine facilitates the CC coupling, while piperidine and diethylamine promote the C-0 coupling. The C-C coupling mechanism of 2,6-dimethylphenol and copper nitrate-acetonitrile catalytic system was deeply studied.
People have discovered that many metal enzymes can be used as catalysts for biological oxidation reactions in nature, so recently many researchers have also applied metal enzyme catalysts to oxidative coupling reactions. In 2003, Anna M. Schuitema synthesized four pyrazole-containing dinuclear macrocyclic copper ligands as catalysts for the oxidative coupling of 2,6-dimethylphenol to obtain diphenoquinone (DPQ) and poly(1,4-ylidene). Phenyl ether) (PPE).
In 2005, Mieke Huisman et al. synthesized several bicyclic copper ligands containing thiophenyl groups as catalysts and obtained PPE and DPQ by passing oxygen, but the amount of diphenoquinone was less than 10%.
Shanghai Tongji University uses 2,6-di-tert-phenol as a raw material to prepare 4,4-biquinone by oxidative coupling, reduction, and de-tert-butyl using p-toluenesulfonic acid as a catalyst. The reaction can be modified to use 2,6-dimethylphenol as a raw material to directly obtain TMBP through oxidative coupling and reduction. This method has simple operation and high yield, which is suitable for industrial production.
Another method is to dissolve 2,6-dimethylphenol in an aqueous solution with a surfactant, pass in oxygen, add a catalyst, and obtain TMBP after post-treatment.
1.2 Using hydrogen peroxide to synthesize TMBP
The use of hydrogen peroxide as oxidant is easy to operate in industry and does not require high equipment. It has a wide range of sources and has a good industrial application prospect. Sagar Sharma et al. synthesized tris(3,5-dimethylpyrazole) copper(II) nitrate, which can catalyze the reaction of 2,6-dimethylphenol in the presence of hydrogen peroxide, with a relatively short time and yield Above 90%. The reaction formula is as follows:
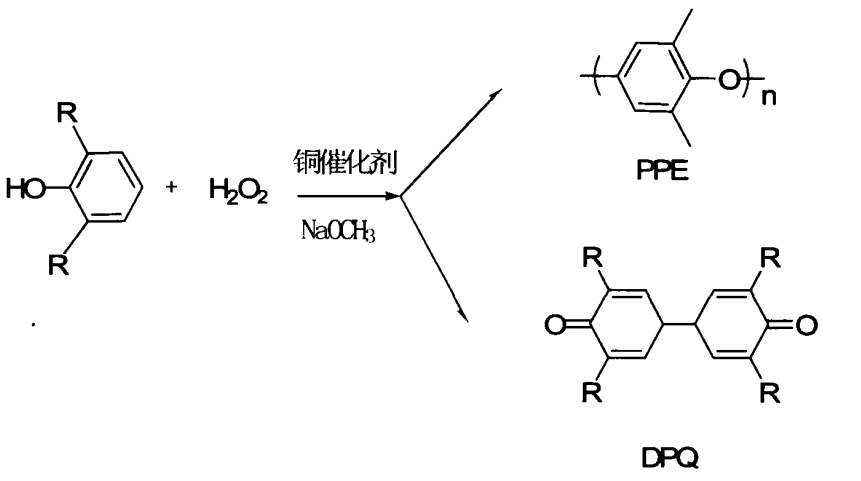
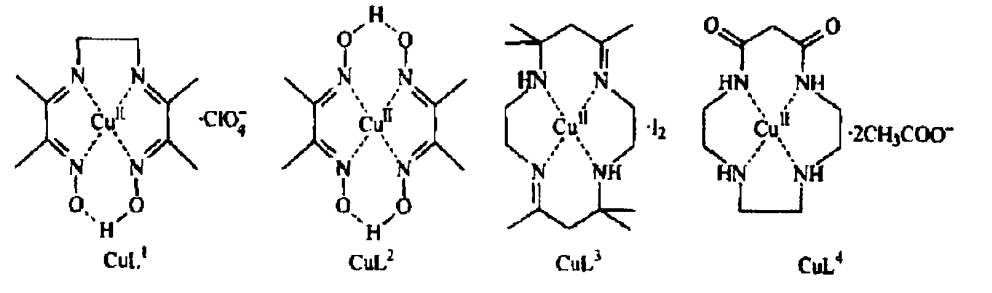
Sichuan University has studied the formation of 3,3′,5,5′-Tetramethyl-4,4′-biphenyldiol from 2,6-dimethylphenol under hydrogen peroxide conditions by synthesizing four kinds of metal copper(Ⅱ)-tetranitrogen complexes. It is proved that this kind of metal complex can catalyze the CC coupling of DMP.
It has been studied abroad that 2,4-di-tertiary phenol and hydrogen peroxide are used as raw materials, and under the conditions of 80℃, alkali and lauric acid as catalysts, 2,2-dihydroxy-3,3, 5,5-Tetra-tert-butyl biphenyl. The target product can be obtained by using this method with 2,6-dimethylphenol as a raw material. In this method, the reactants are easy to obtain, the conditions are simple, and the operation is easy.
1.3 Synthesis of TMBP with inorganic salt oxidation
In 1968, WL. Carrick used VOC13 to synthesize DPQ with a yield of 38%, indicating that inorganic salts can also be used as oxidants for oxidative coupling reactions.
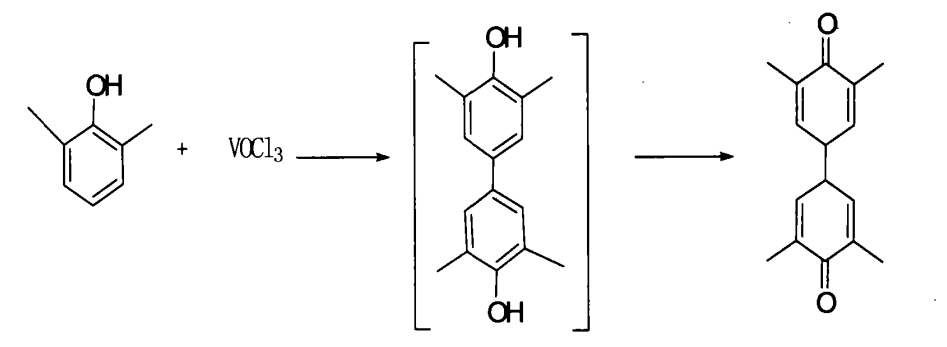
The Guangzhou Institute of Chemistry, Chinese Academy of Sciences uses salt-based oxidants (CuCl2, Ag2C03, Fe2(SO4), etc.) to oxidize and couple to produce DPQ and then reduce to TMBP under acidic conditions with a cheap and efficient inorganic reducing agent. This method has mild reaction conditions and by-products. Less, simple synthesis process, no need to ventilate oxygen, no special reaction device, less equipment investment, and cost saving. Some people also use potassium ferricyanide as an oxidant to also obtain the target product.
1.4 Synthesize TMBP with organic system
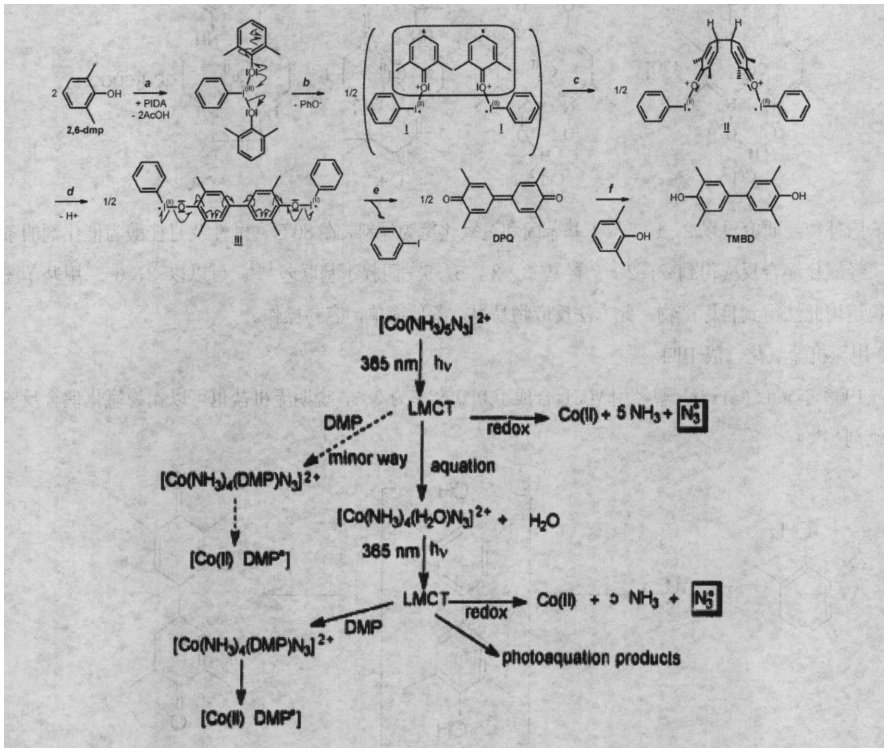
In 2005, Christophe Boldron of Cambridge University studied iodobenzene diacetate (PIDA), dichlorodicyanophosphoquinone (DDQ) and Cu(Ⅱ)/new cuprous reagent (2,9-dimethyl-1,10, phenanthrene). The effect of three oxidants on the reaction products of 2,6-dimethylphenol oxidative coupling. It is concluded that the use of iodobenzene diacetate (PIDA) completely avoids the production of C-0 coupling and directly produces 3, 3′, 5 , 5′-Tetramethyl-4,4-Biphenyldiphenol (TMBD) and 3,3′,5,5′-Tetramethyl-4,4′-Diphenyldiquinone (DPQ). And proposed the mechanism of PIDA oxidation of 2,6-dimethylphenol.
1.5 Synthesis of TMBP by light induction
In recent years, many new technologies have been applied more and more widely in the field of chemistry, such as ultrasonic technology, microwave radiation technology, and optical technology. Therefore, some foreign researchers have used light-induced methods to synthesize TMBP. For example, Mohamed Sarakha and Michele Bolte in France excited (Co(NH)N)+ under 365nm ultraviolet light to achieve the light of 2,6-dimethylphenol. Induced, produced diphenoquinone and TMBP. The application of this method breaks the traditional mode of oxidative coupling and makes the oxidation of 2,6-dimethylphenol a new way.
2. Application
TMBP is an intermediate of biphenyl liquid crystal polymer. Due to its structural particularity, the synthesized polymer has the characteristics of high clearing point and good physical and chemical properties. Like 3,3′,5,5-tetramethylbiphenol diglycidyl ether synthesized by TMBP, it has low melt viscosity, good sealing performance, wide working temperature and no cracking. It is an excellent integrated circuit packaging material. . In addition, TMBP has excellent heat resistance and can also be used as a modified monomer for polyester, polyurethane, polycarbonate, polysulfone, epoxy resin and many other products to produce excellent engineering plastics and composite materials; Oxygen and heat-induced aging can prevent and pollution-free, and can be used as rubber, emulsion antioxidants and plastic antioxidants, as well as dye intermediates or stabilizers for oil products. Such as: can be used for rubber products, food packaging supplies and latex products.
