
Dimorpholinyl diethyl ether (DMDEE, C12H24N2O3) is a strong foaming catalyst. It is a colorless to pale yellow liquid and is soluble in water. It is an amine catalyst suitable for water curing systems. Due to the steric hindrance effect of amino groups, NCO-containing components can have a long storage period. It is mainly used for one-component rigid polyurethane foam systems, and can also be used for polyether and polyester polyurethane soft and semi-rigid foams. , CASE materials, etc.
DMDEE is one of the important polyurethane catalysts, and the main domestic manufacturer is Sinocure Chemical Co., LTD. There are two methods for the synthesis of DMDEE: diethylene glycol and ammonia in the presence of hydrogen and metal catalysts, reacting at high temperature and high pressure to obtain bismorpholinyl diethyl ether; or diethylene glycol and morpholine in hydrogen and metal catalyst copper or cobalt. In the presence of high temperature and high pressure reaction, bismorpholinyl diethyl ether is obtained. The above two synthetic routes both use metals as catalysts and are generally gas-phase high-temperature and high-pressure reactions, which have disadvantages such as high production costs, difficult operation, and difficult product separation. The author uses dichloroethyl ether and morpholine as raw materials in the production of DMDEE, qualified products can be produced under mild conditions, with a product yield of 80% and a product purity of more than 99%.
1.Experimental part
1.1 Main raw materials and instruments
Dichloroethyl ether, mass fraction ≥99%; morpholine, mass fraction ≥99%. The GC6000 gas chromatograph produced by the American Varian Company, with a column of 30 m×0.32 mm × 0.35 μm, produced by the American Dima Company, the stationary liquid is EC-WAX, and the detector is a hydrogen flame detector; the American BIO-RAD Company produces FTS165 Fourier Infrared Spectrometer.
1.2 Synthesis of DMDEE by chloroethyl ether method
Put a certain amount of morpholine into a four-necked flask, drop dichloroethyl ether at a certain temperature and flow rate, and keep it warm for a period of time after the dropwise addition. The reaction is over. Excess morpholine is extracted by vacuum distillation, the remaining solid salt is extracted with cyclohexane, the liquid phase is separated from the extractant and the crude product by atmospheric distillation, and the solid phase is morpholine hydrochloride. The morpholine is recovered by treatment with NaOH, and the crude product is vacuum rectified to obtain DMDEE that meets the quality requirements.
2. Results and discussion
2.1 Reaction process
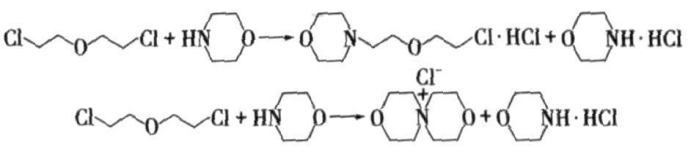
Main reaction:

Side effects:
Main reaction mechanism :
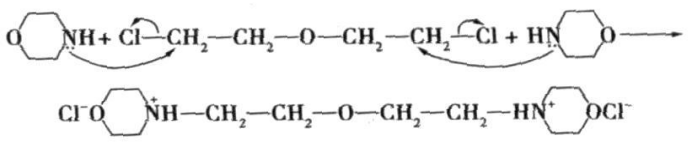
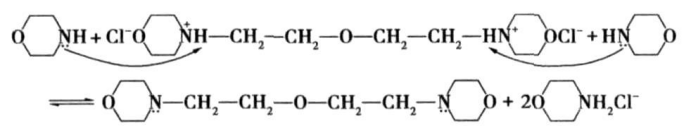
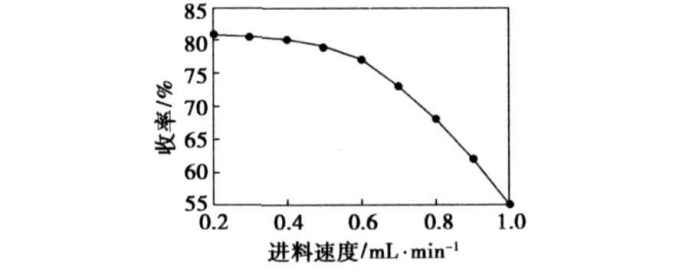
2.2 The influence of the feed rate of dichloroethyl ether on the reaction
Control the feed rate of dichloroethyl ether as follows: 1, 0.9, 0.8, 0.7, 0.6, 0.5, 0.4, 0.3, 0.2 mL/min, take morpholine 300 g, dichloroethyl ether 50 g, and feed temperature 80 ℃ , Detect the final yield of DMDEE, the result is shown in Figure 1. The feed rate is too fast, and the product yield is low. As the feed rate decreases, the product yield gradually increases. When the feed rate drops below 0.4 mL/min, the yield increase is not obvious, indicating that the feed rate is too fast, and the chance of dichloroethyl ether contacting the nucleophile morpholine is relatively reduced, and only one end of the chlorine can interact with it. The morpholino undergoes a nucleophilic substitution reaction, and the resulting monomorpholino-substituted product is more basic, forming its own hydrochloride, which is precipitated in solid form, so that the reaction is terminated and the product yield is reduced. So the best feed rate is 0.4 mL/min.
2.3 The influence of temperature on the reaction
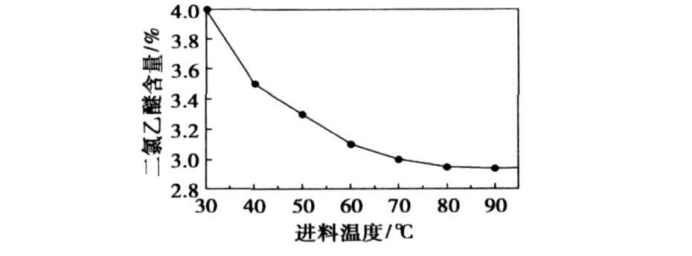
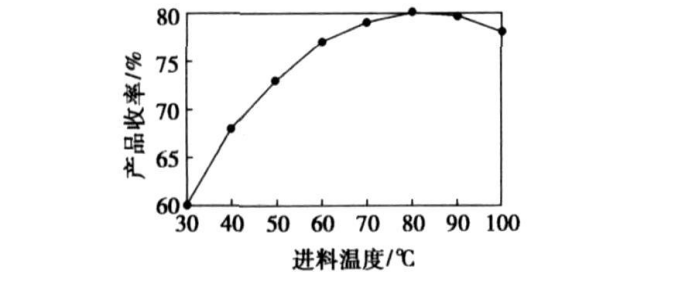
The control temperature is 30, 40, 50, 60, 70, 80, 90, 100 ℃, 300 g of morpholine and 50 g of dichloroethyl ether are taken, and the feed rate is 0.4 mL/min. The final yield of DMDEE is detected, and the After the feed is finished, the content of dichloroethyl ether is detected, and the experimental results are shown in Figure 2 and Figure 3. When the reaction time is the same, the reaction rate of dichloroethyl ether increases with the increase of temperature. But too high temperature can easily cause side reactions, so the best reaction temperature is 80 ℃.
2.4 The effect of the amount of morpholine on the reaction
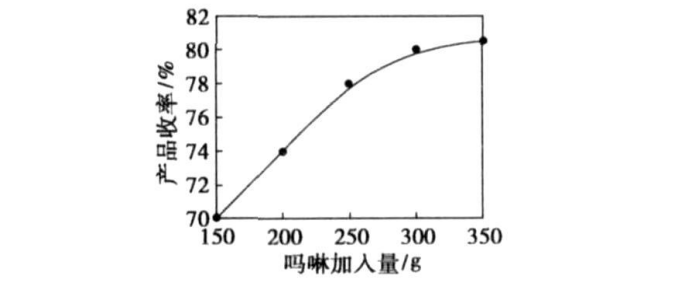
Control the initial amount of morpholine added to 150, 200, 250, 300, 350, 400 g, take 50 g of dichloroethyl ether, feed rate of 0.4 mL/min, feed temperature of 80 ℃, check the final yield of DMDEE , The result is shown in Figure 4. With the increase of the initial amount of morpholine added, the yield gradually increased. However, when the amount of morpholine added exceeds 300 g, this phenomenon is no longer obvious, and it does not make much sense to continue to increase the amount of morpholine, so under the experimental scale, the optimal amount of morpholine added is 300, n (morpholine): n (dichloroethyl ether) =10:1, this is the best material ratio.
Repetitive experiments were carried out with the optimal synthesis conditions obtained from the experiment, the experimental results were stable, and the final yield of DMDEE could be stabilized at about 80%. The synthesized product and the standard product were detected by Fourier infrared spectrometer, and the spectrum of the product DMDEE and the standard product were exactly the same.
3. Conclusion
Dichloroethyl ether and morpholine are used as raw materials to synthesize bismorpholinyl diethyl ether ( DMDEE ). The optimal reaction conditions are: the initial morpholine addition amount is 300 g, the dichloroethyl ether feeding rate is 0.4 mL/min, and the feeding amount 50 g, temperature 80 ℃. Under this reaction condition, the yield of DMDEE after separation treatment can reach 80% (based on dichloroethyl ether). This method has obvious advantages in synthesizing DMDEE, the reaction does not require any catalyst, the reaction conditions are mild and easy to control, the reaction selectivity is high, and there are fewer side reactions, and the basicity of the generated DMDEE is weaker than morpholine, and it does not generate hydrochloride. It exists in the reaction material in the form of liquid, and the product can be obtained after simple separation.
