
In this paper, the photoinitiator system in blue UV curable inks was studied through the compounding of photoinitiators in various ways. The results show that in the composite initiator system, the initiation efficiency of 369/ITX+EDB is the highest. When the initiator BP+P115, which plays the role of surface curing, is added, the curing efficiency can be improved as a whole and the curing time can be reduced.
0 / Preface
UV ink, that is, ultraviolet curing ink, refers to ink that can be converted from liquid to solid and dried under the irradiation of ultraviolet light with a wavelength of 200 to 450 nm. UV ink has the advantages of fast drying speed, high printing production efficiency, low temperature curing energy consumption, high gloss of cured film, good resistance, no solvent volatilization, and environmental protection.
The composition of UV curable ink includes photoactive monomers, photoactive oligomers, photoinitiators, pigments and other auxiliary agents. The photoinitiator is the key part of it. The photoinitiator absorbs ultraviolet light energy and excites to generate free radicals. This triggers the chain reaction of the unsaturated double bonds in the oligomers and monomers in the ink to generate three-dimensional solid substances, which causes the oligomers and monomers to change from liquid to solid. Therefore, the photophysical and chemical properties of the photoinitiator are very important for the control of the photoinitiation and photopolymerization process, and to a large extent determine the speed of photocuring. Since the UV curable ink of the colored system contains pigments, the degree of absorption of ultraviolet light by pigments of different colors is different, which will inevitably cause different photoinitiators of inks of different colors. If the photoinitiator is improperly selected, the light curing speed will be slow, and in severe cases, it will be difficult to cure. Therefore, the choice of photoinitiator system plays a decisive role in UV curing inks. Some workers have done research on the photocurable initiator system, but the literature reports mostly emphasize the use of a single initiator, and some literatures using composite initiators have not done a detailed study on the compounding conditions, and have not fully considered the surface layer. The coordination of curing and deep curing did not find the best initiator system and the best ratio between the initiators. In this paper, taking the blue system as an example, the photoinitiator system in the blue UV curable ink has been studied in detail through the combination of initiators in various ways.
1 / Experimental part
1.1 Raw materials
Low viscosity diacrylate oligomer (CN132), EOEOEA ; tripropylene glycol diacrylate ( TPGDA ), Trimethylolpropane triacrylate ( TMPTA ); Phenylbis(2,4,6-trimethylbenzoyl)phosphine oxide ( Photoinitiator 819 ), 2-Hydroxy-2-methylpropiophenone ( photoinitiator 1173 ), 2,2-Dimethoxy-2-phenylacetophenone ( photoinitiator BDK ), 1-Hydroxycyclohexyl phenyl ketone ( photoinitiator 184 ), 2-Methyl-4′-(methylthio)-2-morpholinopropiophenone ( photoinitiator 907 ), Diphenyl(2,4,6-trimethylbenzoyl)phosphine oxide ( photoinitiator TPO ), 2-Isopropylthioxanthone (photoinitiator ITX), Ethyl 4-dimethylaminobenzoate ( photoinitiator EDB ); P115 ( active tertiary amine ); benzophenone ( BP ); Blue Pigment A.
1.2 Experimental method
The UV absorption spectra of the photoinitiators and pigments were measured using Hitachi U-3010 UV spectrophotometer. Weigh a certain amount of photoactive monomers, oligomers, photoinitiators and pigments according to the formula in Table 1, and disperse them evenly in ultrasonic waves. Roll coated quantitative ink on the film base and cure with 80W/cm UV lamp. The degree of curing is characterized by the finger dry method.
| Sample | Quality score % |
| SR256 | 25 |
| TPGDA | 25.5 |
| TMPTA | 30 |
| CN132 | 13 |
| Blue pigment A | 4.5 |
| Photoinitiator | 2 |
Note: 1. The amount of active amine co-initiator (EDB and P115) is not included in the total photoinitiator
2. The ratio of initiator to co-initiator is 1:1
2 / Results and discussion
Only after the photoinitiator has absorbed enough energy, can it transition to an excited state, and then undergo photochemical and photophysical processes to generate active fragments that can initiate polymerization, such as free radicals and cations. Therefore, in addition to ensuring that the emission spectrum of the light source matches the absorption spectrum of the photoinitiator, the light absorption competition between the pigment in the ink and the photoinitiator must also be considered. Because the pigment has the effects of absorption, reflection, and scattering of ultraviolet light, the intensity of the ultraviolet light irradiated into the film is changed, which affects the efficiency of the photoinitiator, and thus the curing speed of the ink. Therefore, for colored systems, due to the addition of pigments, they have different absorption in the ultraviolet region. Therefore, it is necessary to select the photoinitiator that is least affected by the ultraviolet absorption of the pigment. The weak light absorption band happens to be the strong absorption band of the initiator, that is, it has a “transmission window”.
For the blue ink A system, its ultraviolet absorption spectrum is shown in Figure 1.
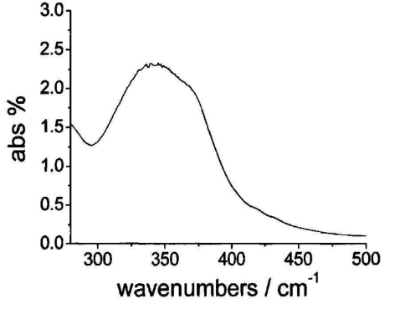
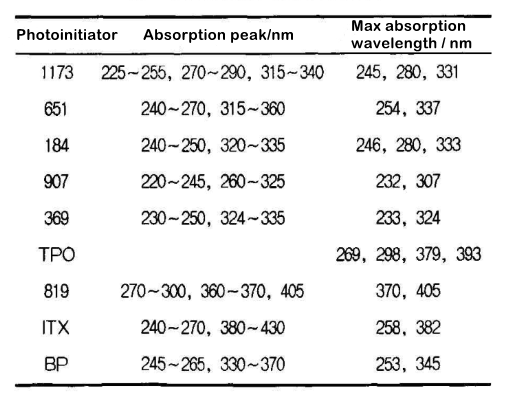
From the UV absorption spectrum in Figure 1, we can see that the blue pigment A has weak absorption at 285~310nm and greater than 380nm, so we have selected the following photoinitiators as a reference, and their absorption peaks and maximum absorption wavelengths As shown in table 2.
First, the curing time of the blue ink under the action of a single initiator was measured, and the results are shown in Figure 2.
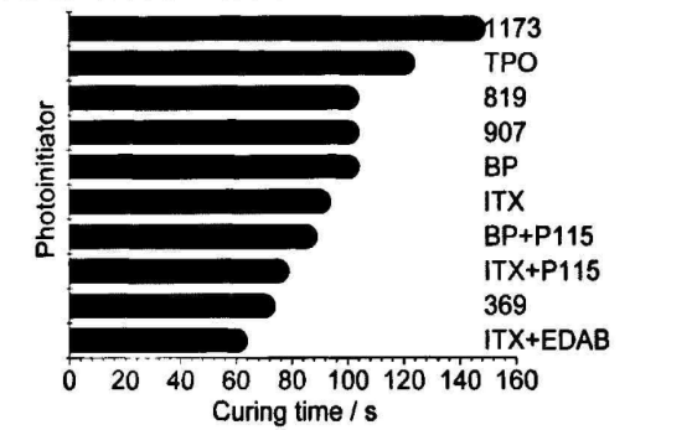
It can be seen from Figure 2 that when a single initiator is used, the curing time of ITX and active amines EDB, BP, P115 and 369 is shorter. The blue pigment A has weak absorption in the wavelength range of 380~450nm, and ITx has a larger absorption in this range. At the same time, the absorption wavelength of ITX and 369 can reach 430nm, which makes its photoinitiation efficiency higher. EDB has higher hydrogen abstraction activity than P115, so the initiation efficiency of ITX+EDB is greater than that of ITX+P115 and ITX. Comparing 369 with 907, although both 907 and 369 belong to a-aminoalkyl phenones and their maximum absorption peaks are relatively close, the para-position of the benzene ring in the structure of 907 is a methyl sulfhydryl group, and the excited triplet lifetime of the initiator is large. Increased, easy to be attacked by strong quenching monomers, and the excited state is quenched, so the curing time is longer than 369.
From Table 2 and Figure 2, we can see that although 819 and TPO also have greater absorption in the blue pigment’s transmission window, the initiation efficiency is not high. This is because although 819 has a high quantum yield, its relative molecular mass is too large, and the free radicals generated under the same mass fraction must be less, so its initiation speed is slow. On the other hand, 819 and TP are both acyl groups. Phosphine oxides, and acyl phosphine oxide initiators are more sensitive to molecular oxygen. Although phosphono radicals have strong addition activity to vinyl monomers, the combination rate of phosphono radicals and molecular oxygen is very fast, so The high addition activity of phosphono radicals does not necessarily guarantee high initiation efficiency, and the addition of active tertiary amines can appropriately alleviate oxygen inhibition.
On the basis of a single initiator, we choose split initiators 369, 907, 819 and hydrogen abstraction initiators ITX+EDB, BP+P115 for compounding. The curing time is shown in Figure 3.
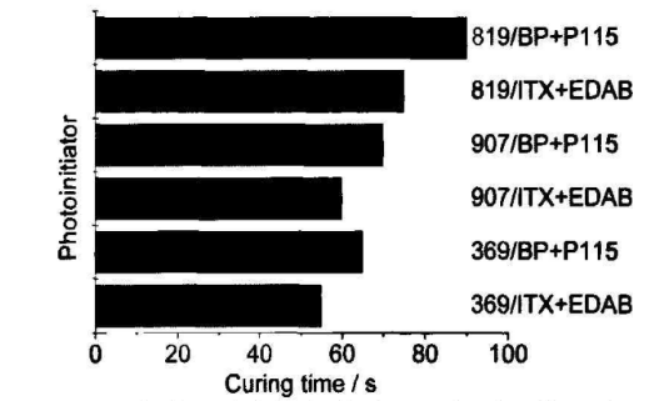
From Figure 3, we can see that the curing time is different for different composite initiator systems. Among them, 369/1TX+EDB and 907/ITX+EDB have the highest initiation efficiency. This is because the composite initiator makes full use of the blue color. The transmission window of the pigment in the short-wave and long-wave regions. ITX has strong absorption at long-wave 380nm, and the absorption wavelength can reach 430nm. 369 and 907 not only have strong absorption in the short-wave range, but also have a certain absorption in the long-wave range. The longest wavelength can reach more than 400nm.
Since both 369 and 907 belong to a-aminoalkyl phenones, this type of photoinitiator has high photoinitiation activity, and in the presence of thioxanthone photoinitiators, its absorption wavelength can reach 380~430nm , Has a higher molar extinction coefficient at 360~405nm, competes with the pigment in the colored system for light absorption, and exchanges energy with a-aminoalkylphenone photoinitiator in its excited triplet state, a-aminoalkylphenone The photoinitiator directly transitions from the ground state to the excited triplet state, indirectly realizes the photoactivation of the a-aminoalkylphenone photoinitiator, and reduces the light absorption of the a-aminoalkylphenone photoinitiator partially shielded by the pigment. Therefore, their combination has a higher initiation efficiency. On the other hand, due to their long-wavelength shift of absorption wavelength, 369, 907 and ITX are also used as initiators for deep curing in colored systems. In order to better improve the initiation efficiency and lower curing time, we added initiators 651, 184, BP+P115 with better surface curing to the above-mentioned composite initiator system. The curing time is shown in Figure 4.
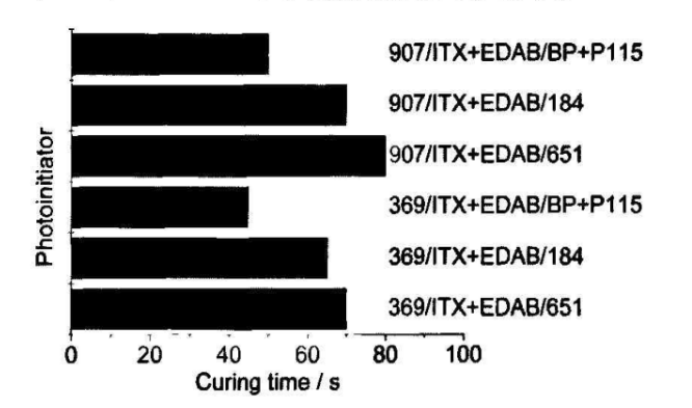
It can be seen from Figure 4 that the curing effect of the surface layer of BP+P115 is better, which can improve the curing efficiency and shorten the curing time as a whole, but it should be noted that the amount of BP+P115 for surface curing should be appropriate. If the effect of surface drying is not achieved, too much will cause the surface layer to cure too fast, which is not conducive to the deep layer curing, so that the surface layer and the inner layer are inconsistent with the curing, and the bottom layer cannot be completely cured or the coating surface has obvious wrinkles.
3 / Conclusion
In this paper, the research on the photoinitiation system of the blue system in UV curing ink shows that: in a single initiation system, the initiation efficiency of 369 and ITX and tertiary amine EDB is higher, and the curing time is shorter; in the composite initiator system, With 369, ITX+EDB has the highest initiation efficiency. When the initiator BP+P115, which plays the role of surface curing, is added, the curing efficiency can be improved as a whole and the curing time can be reduced.

