In the hot summer, in addition to the unbearable heat and the harsh sunlight, people can also put on their favorite sunglasses openly. This is not only fashionable, but also protects the eyes from ultraviolet rays. We all know that sun protection is needed in summer or it will not turn black, but few people know that the eyes need sun protection.
Ultraviolet (UV) radiation from the sun has a wavelength range of 100-400nm, which is shorter than visible light and has a higher frequency and higher energy. According to the wavelength, it can be divided into three groups:
UV A (315-400 nm)
UV B (280-315 nm)
UV C (100-280 nm).
When sunlight travels through the atmosphere, all UV C and about 90% of UV B radiation is absorbed by the ozone layer, water vapor, oxygen, and carbon dioxide. UV A is less affected. Therefore, most of the ultraviolet rays on the earth’s surface are UV A and a small amount of UV B. . The intensity of ultraviolet light can be characterized by the UV index. The higher the number, the greater the harm. If it reaches level 3 and above, it needs protection.

Ultraviolet rays have high energy, so excessive exposure to the sun can cause skin cancer, cataracts and immune system diseases. Ultraviolet rays are as penetrating as visible light, and the lens in the eyeball will age and become cloudy after long-term exposure to ultraviolet rays, which increases the risk of cataracts.
At this time, a pair of sunglasses can block most of the intrusion of ultraviolet rays. So, what is the connection and difference between sunglasses and ordinary glasses?

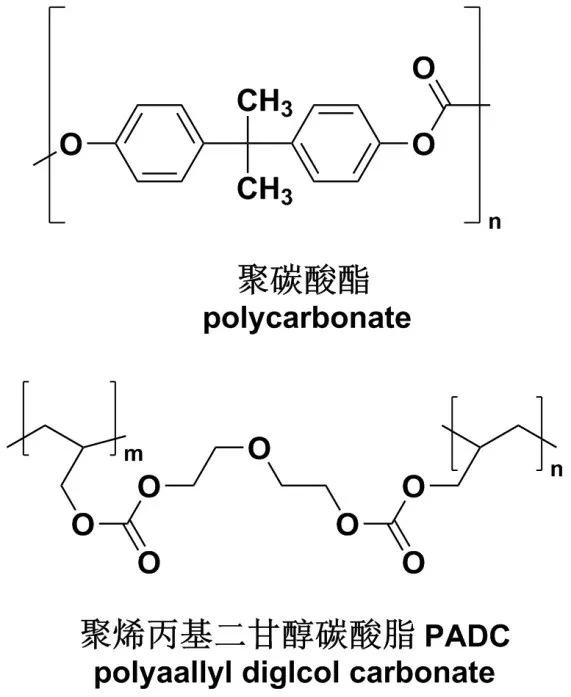
Like other glasses, sunglasses are also made of light-transmitting materials. Commonly used are glass and resin materials, such as polycarbonates or polyaallyl diglcol carbonate PADC, the latter is also called CR -39.

A layer of aluminum or silver is plated on the surface of the glasses to produce a mirror effect. Metal oxide coatings such as silicon oxide, titanium oxide, iron trioxide, manganese dioxide, etc. can reduce the transmittance of ultraviolet rays and protect the eyes.

Another important weapon of color-changing sunglasses is photochromism. Photochromism refers to the changes in the molecular structure of certain compounds under the action of light of a certain wavelength and intensity, resulting in corresponding changes in the absorption peak and color of light, and this change is generally reversible. When there is no ultraviolet radiation, visible light can penetrate these molecules. When exposed to the ultraviolet rays of the sun, these molecules will undergo a reversible change, causing their molecular structure to change, and the new molecular structure will absorb part of the visible light. , So as to darken the lens and reduce the transmittance of ultraviolet rays. When leaving the ultraviolet radiation, these molecules will quickly return to their original structure.

The original color-changing glasses used reversible photochromic materials containing inorganic silver halide (AgX). For example, silver chloride decomposes when exposed to light and turns into many tiny black silver particles, which are evenly distributed in the lens, and the lens becomes dim, hindering the transmission of light. When there is no ultraviolet radiation, under the catalysis of copper ions, silver atoms and chlorine atoms recombine into silver chloride, and the lens becomes transparent again.

Organic photochromic materials, such as naphthopyrans napthopyrans, have good light responsiveness, effective coloration, and better light stability. They can be added to transparent resin lenses and can be used for color-changing sunglasses lenses. The naphthopyran ring opens into cis and trans structures under ultraviolet radiation, and the absorption spectrum of the product changes, such as the absorption spectrum of compounds 3HNP, indeno-NP, 5MR, 6MR, 7MR, etc.
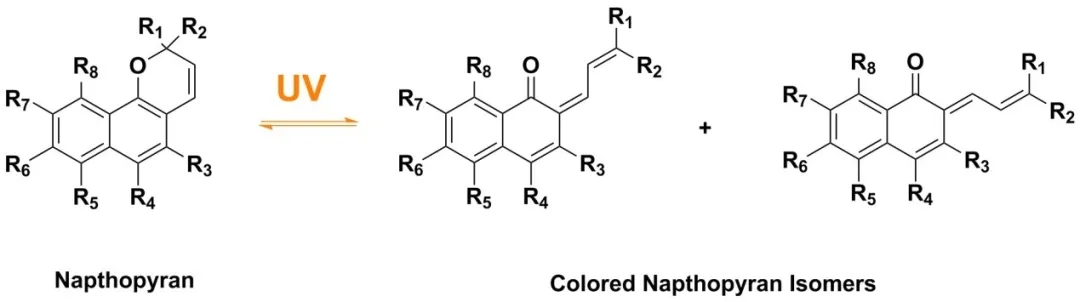
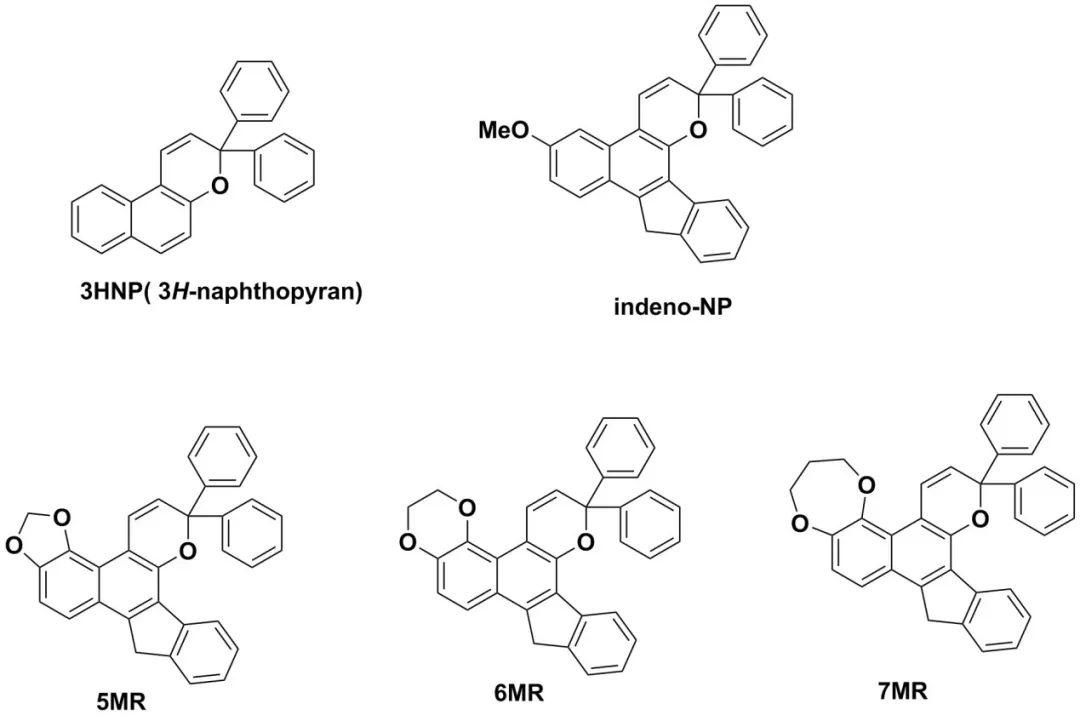
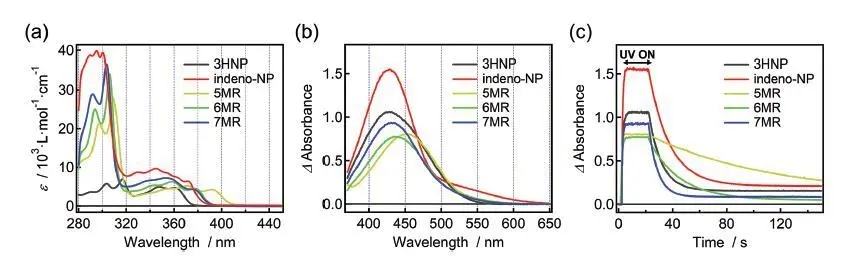
Figure (a) UV absorption spectrum of CFs, (b) single transient absorption spectrum under UV radiation, (c) graph of the change of absorbance at the maximum absorption wavelength with time.
Finally, use pictures to popularize the little knowledge of UV protection: