
Organic reactions are sometimes very mysterious. The conceived reaction process and the reaction results are often different. Sometimes even a side reaction is found, and that side reaction is more valuable than the original reaction; however, most of the time, the life is not good, or the side reaction It’s very complicated, or I don’t know what the side reaction is. As one of the important factors affecting the effect of the reaction, the solvent can sometimes determine the success or failure of a reaction. Therefore, it is necessary for us to have a certain understanding of the reactivity of the solvent, and don’t let the solvent destroy our reaction. Even if it is accidentally found that the reaction involved in the solvent is very meaningful, after understanding the reactivity of the solvent, the result of the reaction can be better explained. This issue will introduce you to those organic reactions that acetone participates in.
Acetone is the simplest saturated ketone, a colorless and transparent liquid, easily soluble in water, methanol, ethanol, ether, dichloromethane, chloroform, pyridine and other organic solvents. It is flammable, volatile, and has more active chemical properties. Therefore, acetone is less used as a solvent in organic reactions. Acetone is generally used as a cleaning solvent in the laboratory. The industrial production of acetone is generally the cumene method. The oxidation of cumene can simultaneously obtain phenol and acetone, which is an important raw material for the industrial preparation of bisphenol A. Acetone is mainly used as a solvent in industries such as explosives, plastics, rubber, fiber, dyeing and finishing, spray painting and other industries. It is also used to synthesize ketene, acetic anhydride, iodoform, polyisoprene rubber, methyl methacrylate, and chloroform. , An important raw material for epoxy resin and other compounds.
01
Acetone reactivity
In the final analysis, acetone is a kind of ketones and has the general properties of ketones. The overall reactivity of acetone can be attributed to two aspects: ① the attack of the nucleophile on the carbonyl group; ② the reaction of the carbonyl group at the α position (electrophilic + free radical).
02
Aldol condensation
The aldol condensation reaction is one of the most basic methods to construct C-C bonds. As early as the 1840s, the Aldol condensation reaction catalyzed by Brønsted acid was discovered. The α-position C-H bond of acetone carbonyl has a certain acidity. It can attack carbonyl-containing compounds in the form of enol or enol anion under acid-base catalysis. Therefore, substrates with carbonyl structure generally do not use acetone as a solvent.
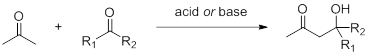
03
Claisen–Schmidt reaction
Aldol condensation reaction produces alcohol. If there is a hydrogen atom on the hydroxyl β carbon, it is easy to eliminate a molecule of water under the action of acid and alkali to obtain α, β unsaturated ketone compound. This is the Claisen–Schmidt reaction.
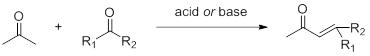
In addition, in the presence of oxidants, primary alcohols can also react similarly with acetone.
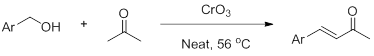
04
Mannich reaction
Mannich reaction, also known as amine methylation reaction, is a three-component reaction of aldehydes and ketones containing alpha hydrogen, formaldehyde and amines.
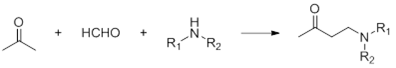
05
Carbonyl α-position halogenation reaction
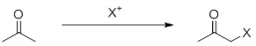
The alpha position of the carbonyl group is more active and can react with NBS, NCS, bromine water, etc.
In addition to these reactions at the α position of the carbonyl group, there are too many addition reactions of nucleophiles to the carbonyl group.
Summary: Since acetone can react with electrophiles and nucleophiles, it is not used as a solvent in organic reactions, but some reactions such as free radical reactions (even acetone can act as a photosensitizer to promote free radicals) Initiation process), pericyclic reaction using acetone as a solvent is particularly good.
